Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at [email protected]
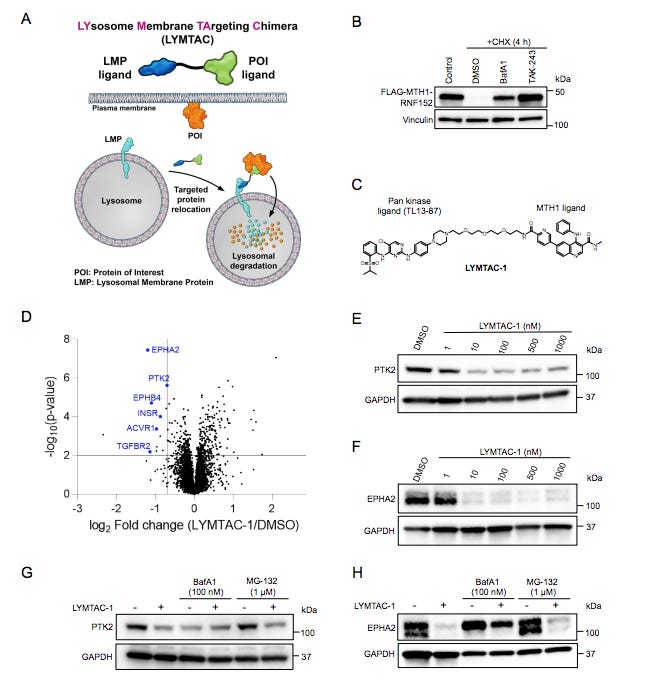
This research invents a new platform called "Lysosome Membrane TArgeting Chimeras" (LYMTACs). These chimeras are heterobifunctional small molecules designed to co-opt short-lived lysosomal membrane proteins (LMPs) as effectors to deliver target proteins for lysosomal degradation.
The authors begin by emphasizing the limitations of traditional inhibitor-based therapies for targeting membrane proteins. Inhibitors often suffer from stoichiometric target inactivation, challenges in target engagement due to a lack of druggable sites, dose-dependent toxicities, and resistance mechanisms. To address these limitations, the field has turned to targeted protein degradation (TPD) techniques, particularly those employing proteolysis targeting chimeras (PROTACs). PROTACs are bifunctional molecules that simultaneously bind to a target protein and an E3 ubiquitin ligase, bringing the target protein into proximity to the ligase, promoting its ubiquitylation and subsequent degradation by the proteasome. While PROTACs have shown great promise in degrading intracellular soluble proteins, their utility for degrading membrane proteins remains less explored due to the challenges associated with lysosomal degradation pathways.
The paper then introduces the concept of LYMTACs as a new approach to address this gap. LYMTACs exploit the unique properties of LMPs, which are short-lived membrane proteins that undergo rapid internalization and degradation via lysosomes. LYMTACs are designed to act as "molecular bridges," bringing together a target protein and a short-lived LMP. This proximity-inducing interaction triggers relocalization of the target protein to the lysosome, leading to its degradation.
The authors demonstrate the proof-of-concept for their LYMTAC technology using a promiscuous kinase inhibitor-based LYMTAC. They successfully induce degradation of multiple membrane protein kinases in HEK293T cells stably expressing a short-lived LMP called RNF152, further validating their approach. Next, they target oncogenic KRASG12D, a challenging membrane protein implicated in various cancers. They design a LYMTAC specifically targeting KRASG12D and demonstrate its ability to induce KRAS ubiquitylation and lysosomal degradation. Notably, they observe that LYMTACs can exert pharmacological activity even in the absence of complete protein degradation, suggesting a dual mode of action that includes both relocalization and sequestration.
The research further delves into the mechanism of action, showing that LYMTAC-induced KRAS relocalization to the lysosomal membrane leads to a deeper suppression of downstream p-ERK signaling compared to conventional KRAS inhibitors. This profound pathway inhibition translates into potent cell killing, highlighting the potential of LYMTACs as an innovative therapeutic strategy. Moreover, the authors showcase the versatility of LYMTACs by demonstrating that multiple LMPs can be utilized as effectors, including LAPTM4a and LAPTM5. This expands the target space and opens up opportunities for targeting a wider range of membrane proteins.
The paper concludes by highlighting the potential of LYMTACs to overcome the challenges of drug resistance often encountered with conventional KRAS inhibitors. Their approach, involving KRAS relocalization and lysosomal degradation, offers a unique mechanism to overcome resistance pathways that rely on KRAS reactivation. Furthermore, the study underscores the multi-pharmacological nature of LYMTACs, highlighting their potential to induce sustained pathway inhibition and potent cell killing compared to single-mode inhibitors.
Overall, this research introduces LYMTACs as a promising new therapeutic platform that addresses the limitations of current drug development strategies. The authors demonstrate the successful application of their technology to target a challenging membrane protein like KRASG12D, opening the door for potentially effective treatments for various cancers. The study showcases the versatility of LYMTACs, suggesting their potential for targeting a wide range of membrane proteins with diverse functionalities, while further emphasizing the importance of proximity-based approaches in drug development.