Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at [email protected]
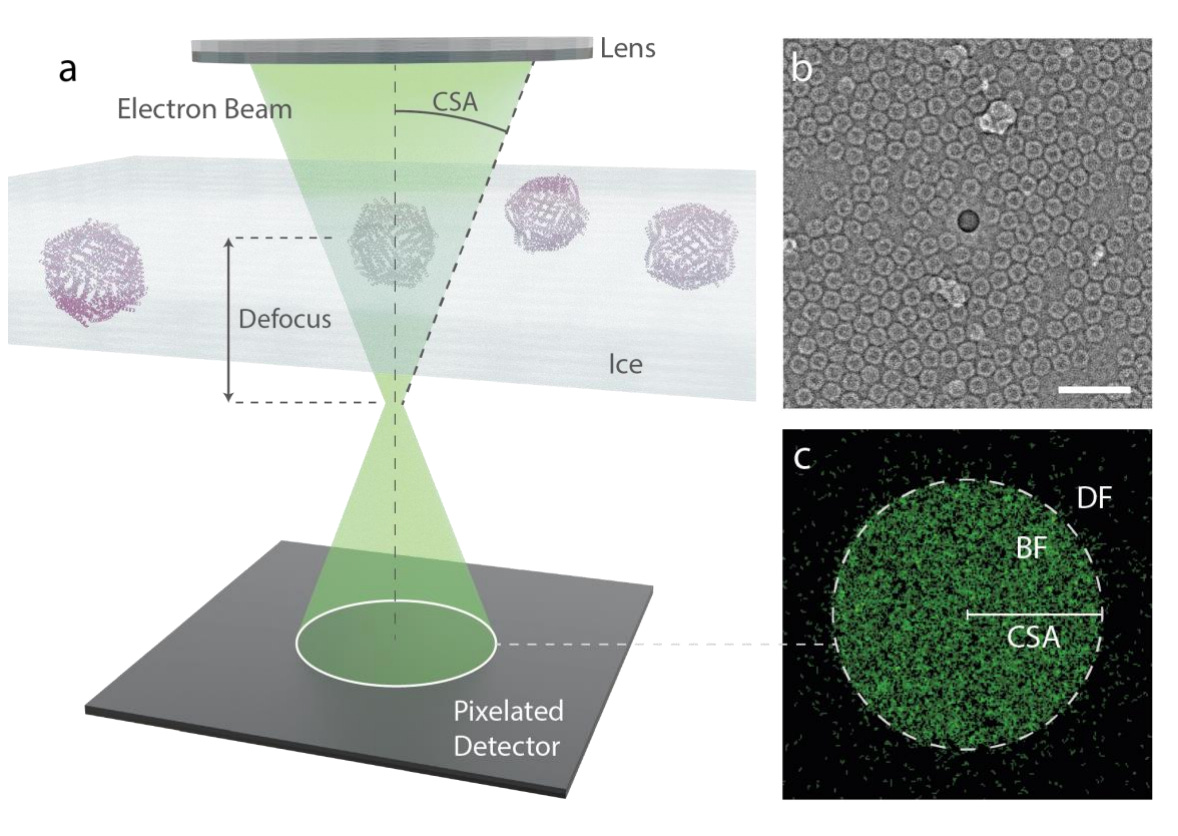
This paper explores the potential of cryo-electron ptychography as a novel method for imaging proteins at sub-nanometer resolutions. Traditional cryo-transmission electron microscopy (cryo-EM) faces limitations in resolving smaller proteins due to limited image contrast. However, cryo-electron ptychography, using a coherent diffractive imaging technique with 4D scanning transmission electron microscopy (4D-STEM), has shown promising results in achieving high resolutions.
The authors applied ptychographic data analysis to frozen hydrated single protein particles, aiming for sub-nanometer resolution 3D reconstructions. They employed low-dose cryo-EM with an aberration-corrected, convergent electron beam to collect 4D-STEM data for their reconstructions. The high-speed electron detector enabled the recording of extensive datasets of electron diffraction patterns with substantial overlaps between interaction volumes. Iterative reconstruction of the scattering potentials from these overlapping patterns showcased strong contrast, enabling a reconstruction of the structure of apoferritin protein at a resolution of 5.8 Å.
The authors investigated the suitability of cryo-electron ptychography for various biomolecules by examining apoferritin, the phi92 bacteriophage, and the tobacco mosaic virus (TMV). They demonstrated that sub-nanometer resolution can be achieved from fewer particles than typically imaged in traditional cryo-EM. This suggests the potential of cryo-electron ptychography for studying smaller proteins or the structural arrangement of smaller features within cells and tissues.
While cryo-electron ptychography shows promise, the authors acknowledge the need for further research and optimization. Key challenges include improving the signal-to-noise ratio in the collected diffraction patterns, especially under low-dose conditions. The authors discussed strategies for minimizing dose constraints and improving data acquisition speed, highlighting the need for advancements in camera technology and data processing algorithms. The paper also highlights the challenges associated with correcting for contrast transfer function effects in ptychography, which are not yet fully established.
The authors suggest that cryo-electron ptychography has the potential to revolutionize structural biology research by providing unique insights into the ultrastructure of vitrified biological tissue. They believe that future research efforts should focus on improving the methods and addressing the limitations of current technology.
In conclusion, the paper presents a compelling case for cryo-electron ptychography as a promising new technique for studying biomolecules at sub-nanometer resolutions. The authors provide a comprehensive overview of the methodology, its advantages, and challenges, paving the way for future research in this exciting field.
The paper meticulously examines the experimental setup and data analysis procedures. They describe the preparation of protein samples, cryo-EM data collection, ptychographic data processing, and the subsequent 3D reconstructions. The authors provide detailed explanations of the algorithms used for particle picking, 2D classification, 3D reconstruction, and resolution estimation.
The paper emphasizes the importance of utilizing a fast hybrid pixel detector for capturing diffraction patterns. They acknowledge the limitations of traditional methods and the need for more efficient data acquisition schemes. The authors also discuss the role of dose fractionation and the importance of addressing the contrast transfer function in ptychography.
The paper highlights the potential of cryo-electron ptychography for studying biological samples, especially for smaller proteins or for visualizing the complex organization of structures within cells and tissues. The authors emphasize the unique advantages of this technique, including the ability to achieve sub-nanometer resolution from a limited number of particles and the potential for exploring thicker biological samples.
The paper concludes by acknowledging the need for further research and optimization. The authors identify key challenges, including improving the signal-to-noise ratio, addressing dose constraints, and developing more robust algorithms for data processing. However, they express optimism about the future of cryo-electron ptychography and its potential to revolutionize structural biology research.
The comprehensive presentation of the methodology, results, and future directions make this paper a valuable contribution to the field of cryo-electron microscopy. It provides a comprehensive overview of the current state of the field, highlighting the challenges and opportunities associated with cryo-electron ptychography. The authors' research efforts contribute significantly to advancing the understanding and applications of this cutting-edge technique.
